In practice, the emitted photo electrons have a range of kinetic energies. Work formula is made use of to compute work done, force, or displacement in any problem.
Equation For Work Function. Work formula is made use of to compute work done, force, or displacement in any problem. In practice, the emitted photo electrons have a range of kinetic energies.
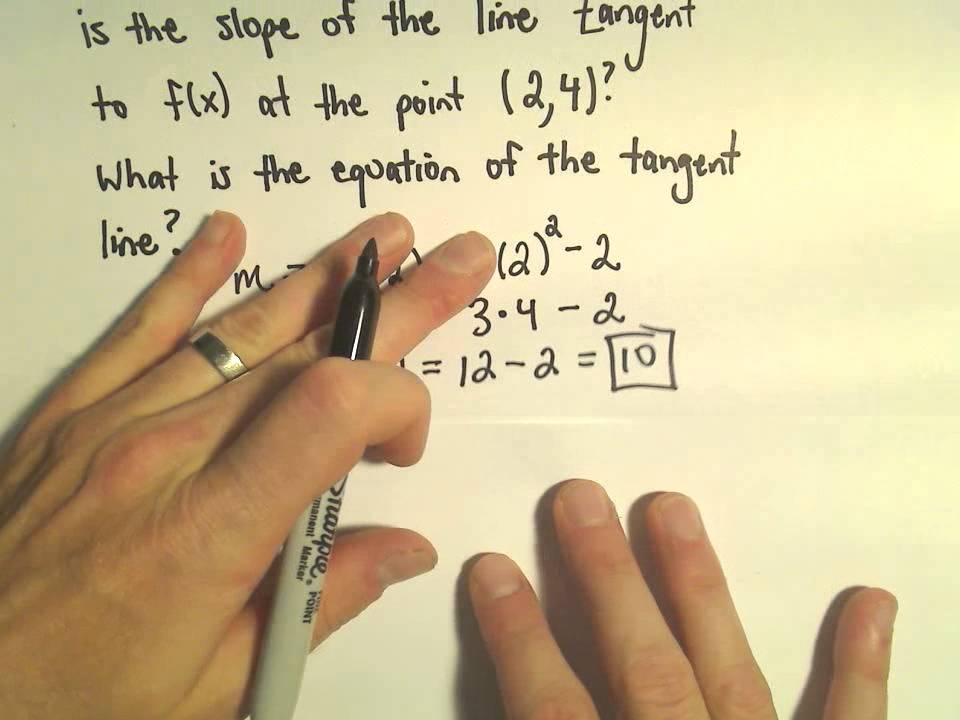
 Finding the Equation of a Tangent Line Using a Derivative From youtube.com
Finding the Equation of a Tangent Line Using a Derivative From youtube.com
If (c2 = yes, then return a 1, otherwise return a 2) =if (c2=1,”yes”,”no”) in this example, the formula in cell d2 says: The work function is the threshold energy, which you would be able to find only if you were able to. If you�re finding the energy of the photon given frequency or wavelength, you would use e = hv.
Finding the Equation of a Tangent Line Using a Derivative
1 9 ) = 3. If (c2 = 1, then return yes, otherwise return no) as you see, the if function can be used to evaluate both text and values. Let�s explore what work function is, and why, in photoelectric effect, electrons come out with different kinetic energies. If (c2 = yes, then return a 1, otherwise return a 2) =if (c2=1,”yes”,”no”) in this example, the formula in cell d2 says:
 Source: youtube.com
Source: youtube.com
In equation form, this is given by kee = hf − be, where kee is the maximum kinetic energy of the ejected electron, hf is the photon�s energy, and be is the binding energy of the electron to the particular material. =if (c2=”yes”,1,2) in the above example, cell d2 says: Work function is the minimum energy needed to remove an.
 Source: youtube.com
Source: youtube.com
Notice that evaluating a function is done in exactly the same way in which we evaluate equations. Work formula is made use of to compute work done, force, or displacement in any problem. The formula of threshold frequency is w= hv 0. Here is f (4) f ( 4). Complete step by step answer:
 Source: youtube.com
Source: youtube.com
In equation form, this is given by kee = hf − be, where kee is the maximum kinetic energy of the ejected electron, hf is the photon�s energy, and be is the binding energy of the electron to the particular material. Complete step by step answer: 6 × 1 0 − 1 9 ) ( 0. And i know the.
 Source: youtube.com
Source: youtube.com
Thankfully, ashcroft and mermin�s classic book has a long discussion on the work function in chapter 18. It also explains how to use the work function of metals to calculate the threshol. There is no term like the threshold frequency of metals because threshold frequency is the characteristics of the electromagnetic radiations and not the metal surface. 1 9 ).
 Source: styrowing.com
Source: styrowing.com
Here is f (4) f ( 4). However, the methods used to solve functional equations can be quite different than the methods for isolating a traditional variable. In equation form, this is given by kee = hf − be, where kee is the maximum kinetic energy of the ejected electron, hf is the photon�s energy, and be is the binding.
 Source: documento.mx
Source: documento.mx
Where , ν o is threshold frequency. Each functional equation provides some information about a function or about multiple functions. Metal on si · fbn tends to increase with increasing metal work function spring 2007 ee 130 lecture 10, slide 10. If (c2 = 1, then return yes, otherwise return no) as you see, the if function can be used.





